Clean without Compromise
Updates from Luma. Non-toxic essentials for a cleaner, worry-free home and life.
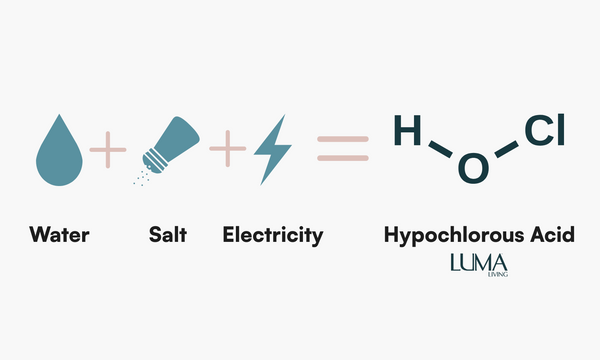
Luma uses hypochlorous acid (HOCl) as our only ingredient. It's a naturally occurring molecule produced by white blood cells to fight viruses and bacteria.
We recreate it through a process called electrolysis, combining salt and water, and running an electrical current through it. The result is HOCl, a non-toxic cleaner for everyday use that's safe for skin and surfaces.
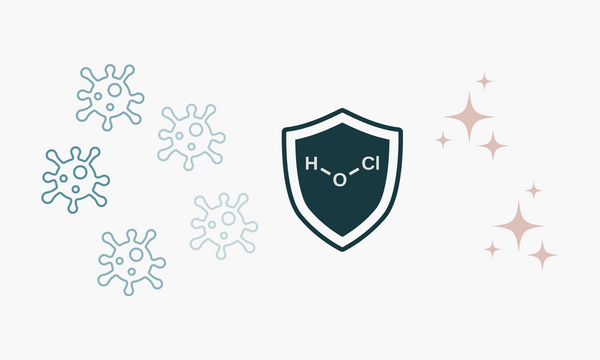
HOCl is electrically neutral, allowing it to penetrate microbial cell walls and neutralize them effectively. It oxidizes harmful pathogens without harming human tissue. That's why it's used in wound care, eye drops, and veterinary disinfection.
Our solution is infused with nano-bubbles, allowing our solution to stay stable and effective for a longer period of time, and penetrate into microscopic crevices in surfaces to ensure a deep, thorough clean.
These studies relate to the ingredient hypochlorous acid in general and are not specific to Luma